Data
1. Clinical Evidence
[ 11 ] Winstanley et al, 2018
A retrospective database analysis of 3792 cases of catastrophic hemorrhage in military major trauma patients on the battlefield was conducted. When comparing patients, 317, who had a hemostatic dressing applied (either Celox (OMNI-STAT), Hemcon or Quikclot) versus no hemostatic agent, results showed there was a 7% improvement in survival. Celox (OMNI-STAT) was the only individual hemostatic dressing that was associated with a statistically significant improvement in survival, which was most apparent in the more severely injured.
Click here to view the full Winstanley publication
[ 9 ] Thibodeaux et al, 2020
|
Study Design
A prospective evaluation of OMNI-STAT was conducted in the OR (52 patients) to compare the effectiveness of OMNI-STAT Granules vs the retrospective use of electrocautery (89 patients) for bleeding control in patients undergoing surgical wound debridement.
Results
OMNI-STAT was shown to be as effective as electrocautery in achieving hemostasis. Significant time savings in the OR was also found, reducing mean total OR time by 19.1%. The results also showed that wounds treated with OMNI-STAT demonstrated a more advanced stage of healing, which may be a result of the lack of tissue damage seen relative to electrocautery. The study concluded that the improved OR times may translate into increased cost effectiveness, relative to electrocautery, by increasing the number of surgical cases per day and/or using resources more effectively to treat a greater number of patients. It may also allow for bleeding control in an outpatient clinic or bedside, which could free up OR time and enable more effective management of healthcare resources.
|
 |
Click here to view the full Thibodeaux publication
[ 3 ] Snyder et al, 2013
The presence of devitalized tissue in wounds may impair the healing process and removal of this tissue promotes wound healing [ 12 ]. The removal of devitalized tissue can be achieved by debridement and sharp debridement removes this necrotic tissue down to the level of well-vascularized tissue [ 13 ]. Sharp debridement can increase the risk of bleeding and this risk is exacerbated by the use of anticoagulants in the aging population where chronic wounds are particularly prevalent. Clinical evaluations have been reported demonstrating positive outcomes of using OMNI-STAT in providing effective hemostasis after sharp debridement, including patients receiving anticoagulants.
Open-label, controlled evaluation (n=40) evaluating chronic wounds of varying etiology with or without
concomitant use of anticoagulants.
| [ 3 ] Snyder and Sigal reported on the results of an open-label, controlled clinical investigation designed to evaluate the hemostatic properties of the chitosan-impregnated gauze, OMNI-STAT, in patients with open wounds of various aetiologies (including diabetic foot ulcer and venous leg ulcer) post-debridement. Forty patients were recruited into the study (n=20, OMNI-STAT; n=20, standard of care). Both groups included a large proportion of patients on anticoagulant therapy (15/20 OMNI-STAT, 16/20 control). After debridement, excess blood was wiped away and the wound was then treated with the OMNI-STAT or the control gauze followed by pressure for 2 minutes. The dressings were left in place for 7 days and then removed at a follow-up visit after being saturated with saline for five minutes. The primary endpoint in the study was the time required to achieve hemostasis with secondary endpoints being an assessment of the patient’s pain levels at the start and end of the treatment and an assessment of the wound bed. |

Time to hemostasis (minutes) [ 3 ]
|
| The mean time hemostasis in the OMNI-STAT treated group was 1 minute 19 seconds compared with 5 minutes and 19 seconds for the control group (p<0.0001). The quality of the granulation tissue of the wound after 1 week was significantly improved in the OMNI-STAT treated group (OMNI-STAT group: 18/20 improved, none deteriorated; control group: none improved, 4/20 deteriorated; p<0.05). Pain scores in the OMNI-STAT group were consistently lower compared with the control group upon application, during application and on dressing removal after one week. The authors conclude that, as well as promoting hemostasis leading to reduced treatment time, the OMNI-STAT provided a moist wound environment and improved the quality of the wound granulation tissue. The establishment of a moist environment may be due to the absorbent nature of the chitosan granules component of OMNI-STAT. The authors suggest that the use of chitosan-impregnated hemostatic dressings may lead to an alteration in clinical practice, allowing for the rapid control of bleeding after wound debridement in patients with chronic wounds despite the prevalence of anticoagulants. |

Tissue quality after 1 week [ 3 ]
|
Click here to view the full Snyder publication
Post Market Clinical Survey Reports
Assessment of real world performance of Celox TM Hemostat Device Family by trained emergency responders in 2022
CELOX Hemostat Devices achieved hemostasis after the first application in 99.3% of cases carried out by respondents including ER physicians, medics, nurses and other emergency personnel. [ 41 ]
The study covered 292 cases involving single or multiple wounds ranging from cutting / piercing and blunt trauma to gunshots and RTAs – 85% of which were described as involving ‘moderate to severe bleeding’.
There were no adverse events for any of the products including those used to treat patients on anticoagulation therapy.
|
Key Findings
- 99.3% success rate, 290/292 cases achieved hemostasis after the first application – even those involving the most severe bleeding cases
- 100% success rate in cases using CELOX Rapid, the most used product
- 100% success rate on safety: no adverse events reported when using any of the products
- 99% of respondents across the whole study rated the CELOX TM family of Hemostat Devices ‘good’ or ‘excellent’
Conclusion
Clinical performance of the CELOX range proves to be highly successful in addressing multiple wounds in the hands of multiple clinical personnel controlling moderate to severe bleeding. 99.3% of the survey participants said hemostasis was achieved after the first application – even in cases involving the most severe bleeding. Every responder using CELOX Rapid said it was 100% successful, and 99% across the survey rated the CELOX family ‘good’ or ‘excellent’ with a 100% safety record; the survey conclusion is a clear endorsement of the effectiveness of CELOX in clinical practice – particularly the highly pressured and demanding environment of the Emergency Department.
|

Wound severity was calculated based on an NISS score
|
CELOX PMFC SURVEY SUMMARY
Assessment of real-world performance of CELOX™ Rapid by emergency physicians and trained emergency responders 2022
CELOX™ Rapid achieved hemostasis after the first application in 100% of cases carried out by respondents including ER physicians, medics, nurses, and other emergency personnel.
This sub-analysis from a larger CELOX™ 292 study covered 84 cases involving single or multiple wounds ranging from cutting/piercing and blunt trauma to gunshots and road traffic accidents – 90% of which were described as involving ‘moderate to severe bleeding’, 21% of which were on anticoagulation therapy.
There were no adverse events recorded.
|
Key Findings
- 100% success rate, 84/84 cases achieved hemostasis after the first application using CELOX™ Rapid – even those involving the most severe bleeding cases
- No adverse events reported when using CELOX™ Rapid
- All respondents across the sub-analysis rated CELOX™ Rapid ‘good’ or ‘excellent’
Conclusion
Clinical performance of CELOX™ Rapid proves to be highly successful in addressing multiple wounds in the hands of a wide range of clinical personnel needed for controlling moderate to severe bleeding. 100% of survey participants of this sub-analysis said hemostasis was achieved after the first application – even in the cases involving the most severe bleeding.
Every responder using CELOX™ Rapid said it was 100% successful, and 100% rated CELOX™ Rapid ‘good’ or ‘excellent’, with a 100% safety record.
The conclusion drawn from the sub-analysis strongly supports the efficacy of CELOX™ Rapid in real world clinical settings, especially in the high pressure and challenging conditions of the Emergency Department. CELOX™ Rapid is proven to control moderate to severe bleeding.
|

Wound severity was calculated based on an NISS score
|
CELOX RAPID FDA PMFC SURVEY SUMMARY
2. In Vivo Studies
Uncontrolled Hemorrhage
|
[ 14 ] Kozen, et al 2008
A standard industry model of a complex groin injury with transection of the femoral vessels and 3 minutes of uncontrolled bleeding was created in 48 swine.
Results show that OMNI-STAT significantly improved hemorrhage control and survival.
|
|
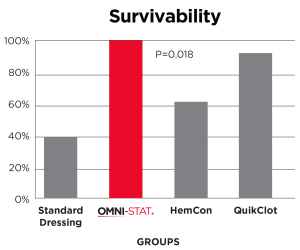
OMNI-STAT improved survival to 100%
|

OMNI-STAT reduced re-bleed to 0%
|
|
|
[ 15 ] Rall, et al 2013
In independent in-vivo test conducted, subjects treated with Celox (OMNI-STAT)* Gauze demonstrated the highest rate of observed survival with 90% when compared to the current standard of care, Combat Gauze demonstrated a 60% survival rate. Celox (OMNI-STAT) also has a substantial history of use on the battlefield (with conventional and special forces) and has repeatedly proven itself in austere settings.
The military's Committee on Tactical Combat Casualty Care (CoTCCC) has added Celox (OMNI-STAT) Gauze to its guidelines for control of hemorrhage as approved hemostatic agents for military-wide use.
*OMNI-STAT and Celox are brand names of equivalent proprietary chitosan technology. For more information on Military & Civilian use visit www.celoxmedical.com
|
  |
OMNI-STAT Clinical and Scientific Monograph
Coagulopathic Bleeding
Millner et al examined the effectiveness of two chitosan derived hemostatic agents (OMNI-STAT granules and OMNI-STAT Gauze) in a porcine model of major hepatic injury in the presence of impaired clotting [ 2 ]. Read more.
3. In Vitro Studies
| Standardized laboratory studies have been used to show the effectiveness of OMNI-STAT and Celox as hemostats. The time taken to form a good clot using freshly drawn blood was assessed and recorded. OMNI-STAT significantly reduced the clotting time compared with the control tests (30.5 secs vs 816.5secs, respectively). The clots were removed from the test article and photographed after 20 minutes, OMNI-STAT showed a robust gel-like clot. [ 1 ] |

Blood clots after 20 minutes [ 1 ]
|
| Studies examining the hemostatic effect of the chitosan derived dressing on coagulation of anticoagulant treated blood showed that hemostasis is largely unaffected by the presence of anticoagulants. Whereas anticoagulant treated blood showed significant delays in coagulation in the standard laboratory tests, the presence of chitosan derived material led to coagulation times similar to those seen in the non anticoagulant treated blood. In this model the heparinised blood clotted in 48s and warfarinised blood in <30s when in contact with OMNI-STAT granules. [ 1 ] |
|

"OMNI-STAT has been shown to stop bleeding in as little as 30 seconds." [ 1 ]
|

"OMNI-STAT has been proven effective even in the presence of anti-coagulants, such as: Heparin™, Coumadin™ (Warfarin)." [ 1 ]
|
|
| OMNI-STAT was able to clot freshly drawn heparinized blood that has been cooled to a temperature of 56°F. The mean clotting time for the OMNI-STAT treated blood was 20 seconds, whereas control blood failed to clot within the 10 minute test period [ 8 ]. |

OMNI-STAT on hypothermic blood
|
References
Please click here to view a list of references.
















